Success in Oncology Projects
Long cooperation between EA and investigators in global cancer centers and oncology networks facilitates
• Fast patient recruitment
• high quality data
Management of long-term studies by the same EA core team (e.g. 8 year long Non Hodgkin lymphoma trial) strengthens our relationship with the participating sites and leads to one-time, on-budget studies.
Performance of studies for targeted
oncological therapies
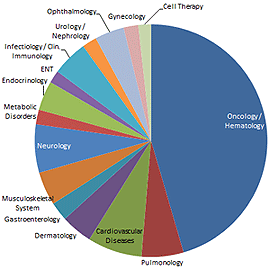
| Therapeutic Area |
No of Studies |
| Cardiovascular Diseases |
34 |
| Dermatology |
13 |
| Ear, Nose, Throat |
5 |
| Endocrinology |
22 |
| Gastroenterology |
19 |
| Immunology |
3 |
| Infectiology |
13 |
| Metabolic Disorders |
6 |
| Muscoloskeletal System |
15 |
| Nephrology |
11 |
| Neurology |
35 |
| Nutrition/Nutraceuticals |
12 |
| Obstetrics & Gynaecology |
7 |
| Oncology/Hematology |
86 |
| Ophthalmology |
24 |
| Respiratory |
18 |
| Urology |
2 |
| Anti-Inflammatory |
1 |
| Vaccines |
3 |
| Infectious Diseases |
2 |
| Other |
2 |
| Total |
333* |
| *including |
No of Studies |
| Diagnostic Imaging |
35 |
| Medical Devices |
14 |
| Pediatric |
19 |
| Nutrition |
11 |
| Stem Cells Therapy |
11 |
|
|
Case Studies
Diagnostic Imaging - Quality
EA enrolled 902 patients for a Phase III Breast Cancer study in 14 countries across Europe, Asia and Americas. The enrollment was completed and the sponsor successfully passed the FDA audit.
Pulmonology - Continuity
A six year, full-service COPD trial was managed by the same EA core team for the entire project!
EA provided the majority of the 572 trial sites from western and eastern Europe, including the highest enrolling sites.
Marketing renewal from the German regulatory authority was given based on this trial.
Immunology - Speed
In a Phase III study for inflammatory autoimmune disease EA completed patient enrollment in 11 months, 7 months faster than the original estimate of 18 months.
Women’s Health - Oversight
EA successfully performed a series of three parallel hormone replacement therapy studies (1 safety study including biopsy, 2 efficacy studies) in Germany and CEE with > 1.200 patients at > 180 sites.
Epilepsy - 'First to File' Registration
EA conducted a patient PK study with bio-analysis for an epilepsy drug at 5 sites. The sponsor was given First -to-File registration in USA.
EA Plays Sponsor Role
EA is acting as Sponsor for a group of MAHs in a Phase IV trial which was requested by EMA. The study is performed by EA in 8 countries in Europe, Asia and US in 30 sites with 350 patients planned.
 |